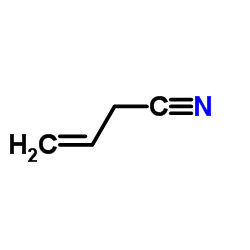
3-butenitrile
CAS NO. 109-75-1
Content: ≥99%
Packaging: 170 kg drum
3-butenenitrile. Its chemical formula is C4H5N and its molecular weight is 67.09 g/mol.
3-Butenenitrile is an aliphatic nitrile and an olefinic compound. It has a carbon-carbon double bond and a carbon-nitrogen triple bond in its structure. It belongs to the class of organic compounds that contain a nitrile group with the general formula R-CN, where R is an organic group.
Physical properties
Some of the physical properties of 3-butenenitrile are listed below:
- Color: clear colorless to yellow liquid
- Shape: linear molecule
- Odor: onion-like odor
- Density: 0.834 g/mL at 25 °C (lit.)
- Melting point: -87 °C
- Boiling point: 116-121 °C (lit.)
- Flash point: 75 °F
- Solubility: not miscible in water; soluble in chloroform and ethyl acetate
- Refractive index: n20/D 1.406
- Vapor pressure: 16.3±0.2 mmHg at 25°C
- Electrical conductivity: no data available
Chemical properties
Some of the chemical properties of 3-butenenitrile are listed below:
- Stability: stable under normal conditions; may polymerize in the presence of metals and some metal compounds
- Reactivity: reacts with acids, strong oxidizing agents, and reducing agents; may form explosive peroxides in air
- Acidity: weakly acidic; pKa = 25.5 (estimated)
- Basicity: weakly basic; pKb = 8.5 (estimated)
- Oxidation-reduction potential: E° = -0.46 V (estimated)
Preparation methods
There are several methods to synthesize 3-butenenitrile, such as:
- Reaction of allyl bromide and cuprous cyanide:
This method can yield 91.3% of 3-butenenitrile with tetrabutylammonium bromide as a catalyst and toluene as a solvent at 60 °C for 6 hours.
- Reaction of allyl chloride and sodium cyanide:
This method can yield 99.5% of 3-butenenitrile with ferrous sulfate as a reducing agent and sodium chloride as a salting-out agent at 60 °C for 6 hours.
- Hydrocyanation of butadiene:
This method can yield 3-butenenitrile selectively with nickel complexes as catalysts and phosphorus-containing ligands as modifiers.
Uses and applications
Some of the uses and applications of 3-butenenitrile are listed below:
- It is used as an anti-microbial agent, as it can release cyanide that inhibits the growth of bacteria and fungi.
- It is used as a pharmaceutical intermediate, as it can be converted into various compounds with biological activities, such as anti-inflammatory, antiviral, antifungal, and anticancer agents.
- It is used as a monomer for polymerization, as it can form polyacrylonitrile and copolymers with other olefins, such as styrene and acrylonitrile.
- It is used as a flavor and fragrance ingredient, as it can impart green, fruity, floral, and spicy notes to various products, such as cosmetics, perfumes, food, and beverages.
Safety and storage
Some of the safety and storage precautions for 3-butenenitrile are listed below:
- It is a flammable, toxic, and irritant substance that can cause severe harm to human health and the environment. It can cause skin and eye irritation, respiratory distress, nausea, vomiting, headache, dizziness, convulsions, coma, and death by inhalation, ingestion, or skin contact.
- It should be handled with care and personal protection, such as gloves, goggles, masks, and clothing. It should be avoided contact with skin, eyes, and mucous membranes. It should be used only in well-ventilated areas or with respiratory protection. It should be kept away from heat, sparks, flames, and other sources of ignition.
- It should be stored in a cool, dry, and well-ventilated place. It should be kept in a tightly closed container. It should be isolated from incompatible materials, such as acids, oxidizers, and reducers. It should be disposed of properly according to local regulations.
